Charlotte Hauser
Brief Biography
Charlotte A.E. Hauser is Professor of Bioscience at King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia. Before joining KAUST, she was a Principal Investigator at the Institute of Bioengineering and Nanotechnology, A*Star, Singapore, and Adjunct Professor at Nanyang Technological University, Singapore. She is a Fellow of the American Institute for Medical and Biological Engineers (AIMBE) and has been elected in 2015 to the rank of NAI Fellow by the US National Academy of Inventors (NAI). After her Ph.D. in Molecular Biology at the Massachusetts Institute of Technology, she joined INSERM in Paris, and the Max-Planck-Institute of Psychiatry in Munich, Germany. Furthermore, she was founder and Managing Director of Octagene in Munich/Martinsried, Germany, where she developed the newest human recombinant coagulation factor VIII (hFVIII), approved in 2014 by FDA, EMEA and other regulatory authorities and available under the trade name of NUWIQ®. Her research includes molecular self-assembly, synthetic peptide biomaterials, amyloidogenesis, and regenerative therapies.
Short Aliphatic Peptide Sequences for Understanding and Preventing Amyloidosis
________________________________________________________________________________________________________
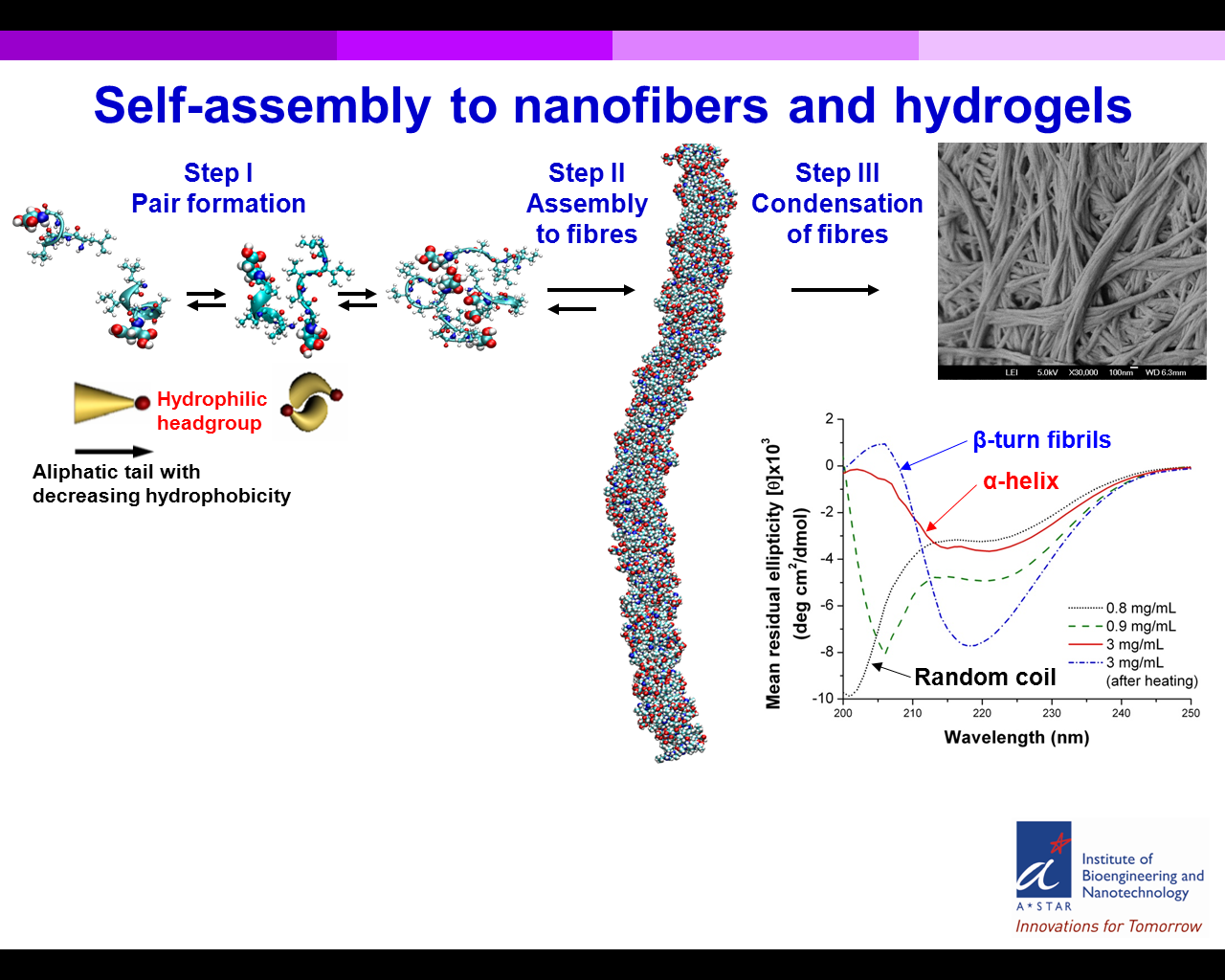
Short aliphatic peptide sequences of just 3-6 amino acids self-assemble in water via α-helical intermediates into amyloid β-type fibers. While core sequences of 4-7 residues that form amyloid fibrils have been identified within natural proteins, the mechanism of aggregation remains unclear. We compared the self-assembly of our designed peptides with core sequences in Amyloid-beta, Amylin and Calcitonin using a multimodal approach. A common feature was the appearance of α-helical intermediates before the final β-turn structures. Another amyloid-beta core sequence containing the diphenylalanine motif was chosen to evaluate the role of aromatic residues in self-assembly. The repeated occurrence of aromatic residues in core sequences has led to widespread conclusions about their key role in driving self-assembly. Surprisingly, the diphenylalanine-containing sequence did not form cross-β aggregates or involve the α-helical intermediate step. Our study puts forth a new, simplified model system to study amyloidosis and indicates that aromatic interactions are not as important as previously postulated. The results provide valuable insight into the early intermediates and factors driving self-assembly, which is necessary for developing small molecule therapeutic drugs that prevent amyloidosis.